Instruction
1
Consider a typical chemical reaction, which is familiar to almost any person. What happens when you make a fire? Organic fuel (in this case, wood), or rather its main component, the carbon enters the oxidation reaction with oxygen in the air. A chemical reaction takes place, accompanied by less abundant evolution of heat, there is a flame. It is written thus:
C + O2 = CO2 Or, for example, the conversion of calcium oxide (quicklime) to calcium hydroxide (slaked lime):
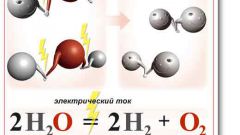
CaO + H2O = Ca(OH)2
C + O2 = CO2 Or, for example, the conversion of calcium oxide (quicklime) to calcium hydroxide (slaked lime):
CaO + H2O = Ca(OH)2
2
You should remember that, unlike mathematical equations, the equations of chemical reactions left and right parts must not be interchanged! Substances in the left part of equation of a chemical reactionare called reactants, and those on the right side – reaction products.
3
It is also necessary to correctly write the formulas of the starting materials and products. Then make sure that this chemical reaction possible, that is, its occurrence does not contradict known physical and chemical laws and regulations. For example, the reaction AgNO3 + NaCl = NaNO3 + AgCl is possible, and the reverse reaction:
AgCl + NaNO3 = NaCl + AgNO3 - no, because silver chloride is almost insoluble. And, despite the fact that the formulas of the substances are written correctly, this reaction is not feasible.
AgCl + NaNO3 = NaCl + AgNO3 - no, because silver chloride is almost insoluble. And, despite the fact that the formulas of the substances are written correctly, this reaction is not feasible.
4
We need to ensure that the number of atoms of each element participating in the reaction, left and right side is the same. This is the main indicator of the correctness of the decision of the equation of the chemical reaction. Example: how to solve an equation of this chemical reaction as the hydrogen reduction of iron from iron oxide of trivalent? Write the starting materials and reaction products.
Fe2O3 + H2 = Fe + H2O
Fe2O3 + H2 = Fe + H2O
5
You will immediately see that the coefficient before the formula of water on the right side of the reaction must be a multiple of 3 (since the left side already has three atoms of oxygen). Check this ratio. You will get:
Fe2O3 + H2 = Fe + 3H2O
Fe2O3 + H2 = Fe + 3H2O
6
By elementary selection, you'll find that in the left and right side of the equation should be: 2 atoms of iron, 3 atoms of oxygen, 6 hydrogen atoms, 3 oxygen atoms. So, the final entry of the equation of chemical reaction is this:
Fe2O3 + 3H2 = 2Fe + 3H2O
Fe2O3 + 3H2 = 2Fe + 3H2O