Instruction
1
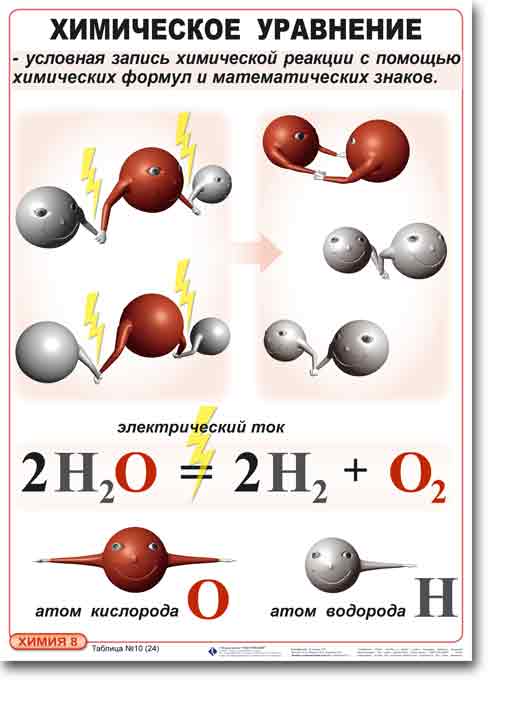
Before proceeding to the task, you need to understand that the figure that is put in front of a chemical element or formula is called a coefficient. And the figure standing after (and beneath) means index. In addition, you need to know that:
• ratio refers to all the chemical symbols standing after it in the formula
• coefficient is multiplied by the index (not sum!)
• the number of atoms of each element reacting substances must be the same number of atoms of these elements included in the composition of the reaction products.
For example, the formula 2H2SO4 means 4 atoms of H (hydrogen) 2 atoms of S (sulfur) and 8 atoms of O (oxygen).
• ratio refers to all the chemical symbols standing after it in the formula
• coefficient is multiplied by the index (not sum!)
• the number of atoms of each element reacting substances must be the same number of atoms of these elements included in the composition of the reaction products.
For example, the formula 2H2SO4 means 4 atoms of H (hydrogen) 2 atoms of S (sulfur) and 8 atoms of O (oxygen).
2
1. Example No. 1. Consider the equation for the combustion of ethylene.
The combustion of organic substances are formed by carbon oxide (IV) (carbon dioxide) and water. Consistently try to place coefficients.
C2H4 + O2 => CO2+ H2O
Begin to analyze. In response to stepped 2 atoms of C (carbon), but it turned out only 1 atom, then put 2 CO2. Now their number is the same.
C2H4 + O2 => 2CO2+ H2O
Now look at H (hydrogen). The response entered the 4 atoms of hydrogen, and was the result of only 2 atoms, therefore H2O (water) put 2 – now turned also 4
C2H4 + O2 => 2CO2+ 2H2O
Consider all the atoms of O (oxygen), formed in the reaction (i.e., after the equal sign). 4 atoms in 2CO2 and 2 atoms in 2H2O – a total of 6 atoms. While reactions of 2 atoms, then a molecule of oxygen O2 set 3, which means that they became too 6.
C2H4 + 3O2 => 2CO2+ 2H2O
Thus, it turned out the same number of atoms of each element before and after the equal sign.
C2H4 + 3O2 => 2CO2+ 2H2O
The combustion of organic substances are formed by carbon oxide (IV) (carbon dioxide) and water. Consistently try to place coefficients.
C2H4 + O2 => CO2+ H2O
Begin to analyze. In response to stepped 2 atoms of C (carbon), but it turned out only 1 atom, then put 2 CO2. Now their number is the same.
C2H4 + O2 => 2CO2+ H2O
Now look at H (hydrogen). The response entered the 4 atoms of hydrogen, and was the result of only 2 atoms, therefore H2O (water) put 2 – now turned also 4
C2H4 + O2 => 2CO2+ 2H2O
Consider all the atoms of O (oxygen), formed in the reaction (i.e., after the equal sign). 4 atoms in 2CO2 and 2 atoms in 2H2O – a total of 6 atoms. While reactions of 2 atoms, then a molecule of oxygen O2 set 3, which means that they became too 6.
C2H4 + 3O2 => 2CO2+ 2H2O
Thus, it turned out the same number of atoms of each element before and after the equal sign.
C2H4 + 3O2 => 2CO2+ 2H2O
3
2. Example No. 2. Consider the reaction of interaction of aluminum with dilute sulfuric acid.
Al + H2SO4 => Al2 (SO4) 3 + H2
Look at the S atoms included in the composition Al2 (SO4) 3 - their 3, and H2SO4 (sulfuric acid) only 1, therefore, before the sulfuric acid is also put 3.
Al + 3H2SO4 => Al2 (SO4) 3 + H2
But now turned to the reaction of 6 atoms of H (hydrogen), and after the reaction is only 2, so, before the molecule H2 (hydrogen) set is also 3, so overall, it was a 6.
Al + 3H2SO4 => Al2 (SO4) 3 + 3H2
Last look at the aluminum. As in Al2 (SO4) 3 (aluminium sulphate) only 2 of the aluminum atom, and to the reaction prior to Al (aluminum) put 2.
2Al + 3H2SO4 => Al2 (SO4) 3 + 3H2
Now the number of all atoms before and after reaction is the same. It turned out that arrange the coefficients in chemical equations is not so difficult. Enough to practice and succeed.
Al + H2SO4 => Al2 (SO4) 3 + H2
Look at the S atoms included in the composition Al2 (SO4) 3 - their 3, and H2SO4 (sulfuric acid) only 1, therefore, before the sulfuric acid is also put 3.
Al + 3H2SO4 => Al2 (SO4) 3 + H2
But now turned to the reaction of 6 atoms of H (hydrogen), and after the reaction is only 2, so, before the molecule H2 (hydrogen) set is also 3, so overall, it was a 6.
Al + 3H2SO4 => Al2 (SO4) 3 + 3H2
Last look at the aluminum. As in Al2 (SO4) 3 (aluminium sulphate) only 2 of the aluminum atom, and to the reaction prior to Al (aluminum) put 2.
2Al + 3H2SO4 => Al2 (SO4) 3 + 3H2
Now the number of all atoms before and after reaction is the same. It turned out that arrange the coefficients in chemical equations is not so difficult. Enough to practice and succeed.
Useful advice
Always keep in mind that the coefficient is multiplied by the index, and do not stack.