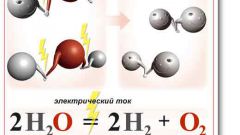
Instruction
1
Correctly write the formula, in accordance with their names. For example, aluminium oxide Al₂O₃, index 3 from aluminum (corresponding to its oxidation number in this compound) put near the oxygen, and the index 2 (oxidation state of oxygen) near the aluminium.
If the degree of oxidation of +1 or -1, the index is not assigned. For example, you need to write down the formula of ammonium nitrate. Nitrate acid residue of nitric acid (-NO₃, S. O. -1), ammonium (-NH₄, p. o. +1). Therefore, the formula of the nitrate of ammonium - NH₄ NO₃. Sometimes the oxidation state is specified in the title compound. Sulfur oxide (VI) - SO₃, silica (II) SiO. Some simple substances (gases) is written with index 2: Cl₂, J₂, F₂, O₂, H₂, etc.
If the degree of oxidation of +1 or -1, the index is not assigned. For example, you need to write down the formula of ammonium nitrate. Nitrate acid residue of nitric acid (-NO₃, S. O. -1), ammonium (-NH₄, p. o. +1). Therefore, the formula of the nitrate of ammonium - NH₄ NO₃. Sometimes the oxidation state is specified in the title compound. Sulfur oxide (VI) - SO₃, silica (II) SiO. Some simple substances (gases) is written with index 2: Cl₂, J₂, F₂, O₂, H₂, etc.
2
You must know which substances react. Visible signs of reaction gas evolution, color change, and sedimentation. Very often, reactions take place without visible changes.
Example 1: neutralization reaction
H₂SO₄ + 2 NaOH → Na₂SO₄ + 2 H₂O
Sodium hydroxide reacts with sulfuric acid to form the soluble salt sodium sulfate and water. The sodium ion is cleaved and connected to the remainder of the acid, replacing the hydrogen. The reaction takes place without external signs.
Example 2: iodoformi sample
SNO + 4 J₂ + 6 NaOH→CHJ₃↓ + 5 NaJ + HCOONa + 5 H₂O
The reaction takes place in several stages. The end result is the deposition of crystals of iodoform yellow color (positive reaction to alcohol).
Example 3:
Zn + K₂SO₄ ≠
The reaction is not possible, because the number of the stress of metals, zinc is second only to potassium and not can displace it from the connections.
Example 1: neutralization reaction
H₂SO₄ + 2 NaOH → Na₂SO₄ + 2 H₂O
Sodium hydroxide reacts with sulfuric acid to form the soluble salt sodium sulfate and water. The sodium ion is cleaved and connected to the remainder of the acid, replacing the hydrogen. The reaction takes place without external signs.
Example 2: iodoformi sample
SNO + 4 J₂ + 6 NaOH→CHJ₃↓ + 5 NaJ + HCOONa + 5 H₂O
The reaction takes place in several stages. The end result is the deposition of crystals of iodoform yellow color (positive reaction to alcohol).
Example 3:
Zn + K₂SO₄ ≠
The reaction is not possible, because the number of the stress of metals, zinc is second only to potassium and not can displace it from the connections.
3
The law of conservation of mass States that the mass of substances, which entered into reaction is equal to the mass of the resulting substances. Proper recording of chemical reactions is half the battle. You need to place the coefficients. Begin to equalize with those of compounds in the formulas which contain large indexes.
K₂Cr₂O₇ + 14 HCl → 2 CrCl₃ + 2 KCl + 3 Cl₂↑ + 7 H₂O
Arrange the coefficients start with the potassium bichromate, because its formula contains the highest index (7).
Such accuracy in the recording of the reactions required for calculation of mass, volume, concentration, of the released energy and other values. Be careful. Remember the most common formulae of acids and bases, and acidic residues.
K₂Cr₂O₇ + 14 HCl → 2 CrCl₃ + 2 KCl + 3 Cl₂↑ + 7 H₂O
Arrange the coefficients start with the potassium bichromate, because its formula contains the highest index (7).
Such accuracy in the recording of the reactions required for calculation of mass, volume, concentration, of the released energy and other values. Be careful. Remember the most common formulae of acids and bases, and acidic residues.