Instruction
1
Remember the multiplication table, if there is no auxiliary computational tools, but there is a need to calculate the square of any number. If this can be done, multiply in the mind or on paper (in a column) you are interested in the number itself. This method of computing nowadays, perhaps, be attributed to the category of intelligent entertainment or gymnastics for the mind, as it is neither the fastest nor the simplest.
2
Use the search engine Google or Nigma, if computational tools are not in your possession, but have access to the Internet. There is no need something to search for in these search engines - they are calculators. Simply enter a relevant query and get the already calculated result. For example, to know the value of the squared number of 5.47 send to the server a search engine query 5,47*is 5.47, or 5, 47^2, or "5,47 squared" - in each case, the search engine will show you the correct answer (29,9209).
3
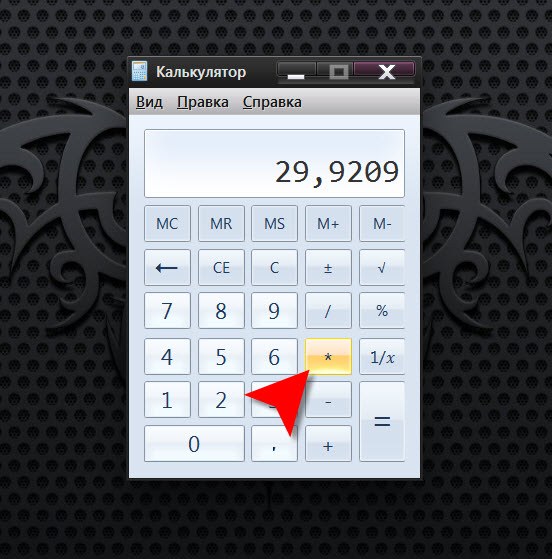
Run the program simulates an ordinary calculator, if described in the previous two steps ways for some reason unavailable. This program is part of the standard Suite of applications installed with the operating system. In Windows any version you can open it using the standard dialog run programs called on the screen by simultaneously pressing Win key and R. the only field of this dialog, type calc and press Enter.
4
Enter the number whose square it is necessary to calculate - just enter it on the keyboard or click the appropriate buttons in the calculator interface. Then type the command multiplication - press the asterisk or click on the same button in the interface. To enter the second times the number required, just press Enter and the calculator will display the result of multiplying the number by itself.