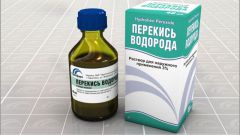
Hydrogen peroxide is the simplest representative of peroxides, it is colorless, has a metallic taste, infinitely soluble in water, alcohol and ether. Peroxide is the most common antiseptic, it disinfects the wounds and symptoms of skin irritation, kills them in all sorts of infection.
Peroxide is the strongest oxidizer, if even a drop gets on any part of human skin, immediately there is a reaction, a white spot appears, which can be considered as a dead site on which there are no viruses and bacteria.
Perhaps the most common explanation for the hiss of hydrogen peroxide is its property in contact to disintegrate and oxidize. And she breaks up with liberation of oxygen, which is accompanied by some hiss. While in the jar, hydrogen peroxide is fairly stable, but it's worth it to react with the blood in the wound, it immediately breaks. Many believe that using foam, which is released when the hissing, go outside and dirt, and microbes.
Hydrogen peroxide contains a small amount of toxic substances that allows its use in all spheres of human life. You will not believe, but there are hundreds of ways of its application. Just imagine, hydrogen peroxide is used:
in teeth whitening,
- in the treatment of acne,
- in surgery, burns,
- when refueling of air conditioners and cleaning the filters
- rocket fuel
- in the food industry.
These areas are not linked, but the hydrogen peroxide used everything. In medicine hydrogen peroxide is used as disinfectant, in the treatment of wounds after accidents, purulent skin, various body lesions, in order to disinfect and to ensure the purity and sterility of the damaged area of the skin. In contrast to the solution of brilliant green, hydrogen peroxide is more compatible with sensitive mucous cells, not burn them and only washes and disinfects. However, as with any active drug, once in the eye, peroxide is irritating, so, you should immediately wash and go to the doctor.
Is hydrogen peroxide got in the fight with sulfuric plugs in ears: a drop of hydrogen into the ear and starts to sizzle, the patient hears the sound, this is probably the most annoying thing in the procedure! Peroxide reacts with sulfuric tube, it just destroys it with its powerful substances.
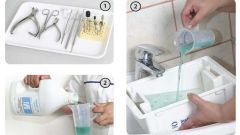
In addition, the hydrogen peroxide used in the processing of common goods (pens, microphones, glasses in movie theaters), and medical equipment: it is poured into special containers in which they place various medical subjects that they undergo complete disinfection. Thus treated product aseptic and safe.
The oxidation reaction
Peroxide is the strongest oxidizer, if even a drop gets on any part of human skin, immediately there is a reaction, a white spot appears, which can be considered as a dead site on which there are no viruses and bacteria.
Hydrogen peroxide, oddly enough, is a form of oxygen.
Perhaps the most common explanation for the hiss of hydrogen peroxide is its property in contact to disintegrate and oxidize. And she breaks up with liberation of oxygen, which is accompanied by some hiss. While in the jar, hydrogen peroxide is fairly stable, but it's worth it to react with the blood in the wound, it immediately breaks. Many believe that using foam, which is released when the hissing, go outside and dirt, and microbes.
A useful poison
Hydrogen peroxide contains a small amount of toxic substances that allows its use in all spheres of human life. You will not believe, but there are hundreds of ways of its application. Just imagine, hydrogen peroxide is used:
in teeth whitening,
- in the treatment of acne,
- in surgery, burns,
- when refueling of air conditioners and cleaning the filters
- rocket fuel
- in the food industry.
These areas are not linked, but the hydrogen peroxide used everything. In medicine hydrogen peroxide is used as disinfectant, in the treatment of wounds after accidents, purulent skin, various body lesions, in order to disinfect and to ensure the purity and sterility of the damaged area of the skin. In contrast to the solution of brilliant green, hydrogen peroxide is more compatible with sensitive mucous cells, not burn them and only washes and disinfects. However, as with any active drug, once in the eye, peroxide is irritating, so, you should immediately wash and go to the doctor.
Hydrogen peroxide is sometimes even taken internally for medicinal purposes.
Is hydrogen peroxide got in the fight with sulfuric plugs in ears: a drop of hydrogen into the ear and starts to sizzle, the patient hears the sound, this is probably the most annoying thing in the procedure! Peroxide reacts with sulfuric tube, it just destroys it with its powerful substances.
In addition, the hydrogen peroxide used in the processing of common goods (pens, microphones, glasses in movie theaters), and medical equipment: it is poured into special containers in which they place various medical subjects that they undergo complete disinfection. Thus treated product aseptic and safe.