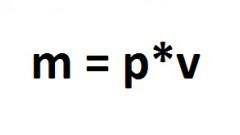You will need
- calculator,
- - Libra,
- - barometer,
- thermometer,
- - Handbook of physics.
Instruction
1
Determine the weight of the substance in grams. To do this, use weights.
Many foods are sold pre-packaged. In this case, the question of the weight disappears by itself. For example, in a standard pack of sugar, this food product is one kilo exactly.
Many foods are sold pre-packaged. In this case, the question of the weight disappears by itself. For example, in a standard pack of sugar, this food product is one kilo exactly.
2
Find it in the Handbook of physics the density of the desired substance.
For more accurate result of subsequent calculations, use an existing reference corrections for pressure, humidity and temperature of the air.
For more accurate result of subsequent calculations, use an existing reference corrections for pressure, humidity and temperature of the air.
3
The value of the density of the material in the Handbook may be given in different units. If the density is specified in kg/cubic m - set the value of the density in g/CC and ml To do this, move the kilograms to gramsand cubic meter - milliliters. Then substitute in the numerator and denominator of the respective units, and they multiply the table value density: the density (from table) * 1000 g/1 000 000 ml.
4
Use the calculator to calculate the amount of material: divide the mass by the density.
Don't forget about the units!
The resulting value will determine the ratio of the mass of a substance in grams by its volume in milliliters.
Don't forget about the units!
The resulting value will determine the ratio of the mass of a substance in grams by its volume in milliliters.
Note
To eliminate the error in the calculations, carefully handle the units.
Useful advice
1 kilogram – 1000 grams. 1 liter – 1000 milliliters.
It is considered that 1 gram of water equals 1 milliliter 1 liter of distilled water at a temperature of + 4oC weighs about 1 kg (density of water under these conditions is 1000 kg/cubic meter). The change in the density of tap water (the ratio of body mass to the volume that it occupies), due to changes in pressure and temperature of the ambient air in the home is usually not taken into account. When more accurate calculations (for example, when determining the mass of ballast on ships) - it takes into account everything.
If we talk about water density - the sea water is heavier than fresh. This is due to the presence of salts. Other substances density is also more or less changes depending on external factors: pressure, humidity and ambient temperature. In this case, the substance is more hygroscopic (absorbs moisture intense), the larger changes in density. That is the same capacity of a certain volume, filled with substances with different density will have different mass. For example, the density of the sand, depending on the maintenance in it of water changes by about half.
For the convenience of users, there are conversion chart grams to milliliters for many food products. On the basis of these tables are made of plastic measuring mug for kitchen liquid and solids.
It is considered that 1 gram of water equals 1 milliliter 1 liter of distilled water at a temperature of + 4oC weighs about 1 kg (density of water under these conditions is 1000 kg/cubic meter). The change in the density of tap water (the ratio of body mass to the volume that it occupies), due to changes in pressure and temperature of the ambient air in the home is usually not taken into account. When more accurate calculations (for example, when determining the mass of ballast on ships) - it takes into account everything.
If we talk about water density - the sea water is heavier than fresh. This is due to the presence of salts. Other substances density is also more or less changes depending on external factors: pressure, humidity and ambient temperature. In this case, the substance is more hygroscopic (absorbs moisture intense), the larger changes in density. That is the same capacity of a certain volume, filled with substances with different density will have different mass. For example, the density of the sand, depending on the maintenance in it of water changes by about half.
For the convenience of users, there are conversion chart grams to milliliters for many food products. On the basis of these tables are made of plastic measuring mug for kitchen liquid and solids.