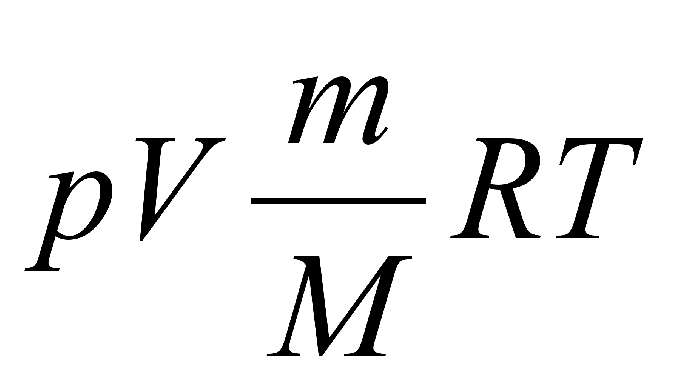You will need
- A sheet of paper, a pen.
Instruction
1
The formula is as follows: p•Vm = R•T where p is the pressure, Vm is molar volume of gas, R is the universal gas constant, and T is the absolute temperature of an ideal gas.
2
Find out what data is available to us in order to use the formula, thus: T = (p•Vm)/ R.
3
If we are not aware of molar volume of gas, we can find it by formula:
Vm = V/?. In this formula ? represents the number of substances to Find this value is possible by dividing the mass of gas to its molar mass.
Vm = V/?. In this formula ? represents the number of substances to Find this value is possible by dividing the mass of gas to its molar mass.
4
The formula, which is called the law of Mendeleev-Clapeyron, is written in this form: p•V = (m/M) • R•T.
5
Modifies this formula to find the temperature of the gas: T = (p•V • M)/(R• m).
6
Find all the values that require us to substitute into the formula. Calculations and find the desired temperature perfect gas.
Note
Carefully examined in the legend, due to a correctly recognized symbol in the formula to prevent the error in the calculations.
Useful advice
The law Mendeleev-Clapeyron also called the combined gas law, that from it are derived the laws of Charles and Gay-Lussac, and Boyle.