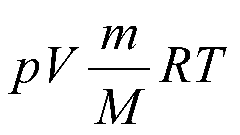Instruction
1
Most common in problems formula: V = n*Vm where V is the volume of gas (l), n – amount of substance (mol), Vm is molar volume of gas (l/mol), at normal conditions(n.) is a standard size and is equal to 22.4 l/mol. It so happens that in condition no of quantity of substance, but there are lots of a certain substance, then do so: n = m/M where m is the mass of substance (g) M – molar mass substance (g/mol). Molar mass find the table of D. I. Mendeleev: below each element is written to its atomic mass, add up all the masses and get. But such tasks are rare, usually in the present problem the equation of the reaction. The decision of such tasks it changes a little. Consider the following example.
2
What volume of hydrogen under normal conditions, if dissolved aluminium weight 10.8 g in excess hydrochloric acid.
Write the reaction equation: 2Al + 6HCl(g) = 2AlCl3 + 3H2.
Solve the problem of this equation. Find quantity of substance of aluminum, which are reacted: n(Al) = m(Al)/M(Al). To substitute data into the formula we need to calculate the molar mass of aluminium: M(Al) = 27 g/mol. Inline: n(Al) = 10,8/27 = 0,4 mol.From the equation we see that by dissolving 2 mole of aluminum is formed 3 mol of hydrogen. Expect what amount of substance of hydrogen is formed from 0.4 mole of aluminum: n(H2) = 3*0,4/2 = 0,6 mol. Then substitute the numbers into the formula for finding the volume of hydrogen: V = n*Vm = 0.6 x 22,4 = 13,44 L. Here we got the answer.
Write the reaction equation: 2Al + 6HCl(g) = 2AlCl3 + 3H2.
Solve the problem of this equation. Find quantity of substance of aluminum, which are reacted: n(Al) = m(Al)/M(Al). To substitute data into the formula we need to calculate the molar mass of aluminium: M(Al) = 27 g/mol. Inline: n(Al) = 10,8/27 = 0,4 mol.From the equation we see that by dissolving 2 mole of aluminum is formed 3 mol of hydrogen. Expect what amount of substance of hydrogen is formed from 0.4 mole of aluminum: n(H2) = 3*0,4/2 = 0,6 mol. Then substitute the numbers into the formula for finding the volume of hydrogen: V = n*Vm = 0.6 x 22,4 = 13,44 L. Here we got the answer.
3
If we are dealing with a gas system that has a formula q(x) = V(x)/V, where q(x)(Fi) is the volume fraction of the component V(x) is the volume of component (l), V is the volume of the system (l). To find the volume component, we obtain the formula: V(x) = q(x)*V. But if you need to find the system volume, then: V = V(x)/q(x).
Note
There are other formulas to find the volume, but if you want to find the volume of gas will only fit the formulas given in this article.