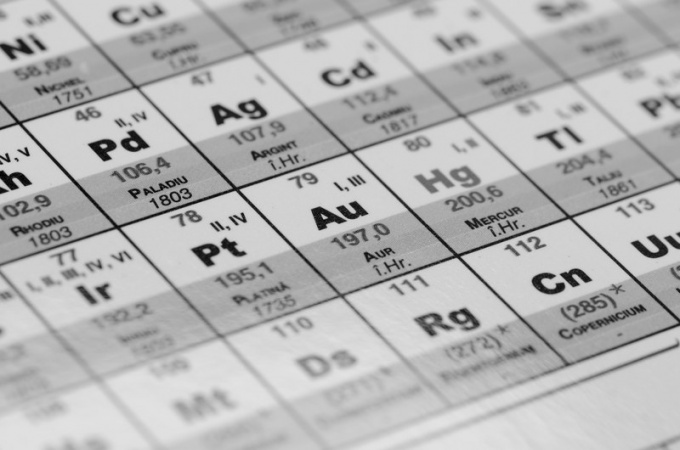
In the XIX century it was believed that atomic mass is the main characteristic of the element, therefore for the classification of substances used it. Now the atoms and identificeret determine the magnitude of the charge of their nuclei (protons and ordinal position in the periodic table). However, atomic mass of elements with some exceptions (for example, atomic mass of potassium is less than the atomic weight of argon) is increased in proportion to their nuclear charge.
With increasing atomic mass and observed periodic variation of properties of elements and their compounds. This metallicity and nemetallicheskie of atoms, atomic radius and volume, ionization potential, electron affinity, electronegativity, oxidation States, physical properties of compounds (boiling point, melting point, density), their basicity, or acidity afternote.
The periodic table graphically expresses which he discovered the periodic law. In the modern periodic table contains 112 chemical elements (last – Materi, Darmstadt, Rentgene and Copernici). According to recent reports, opened and the next 8 elements (up to 120, inclusive), but not all of them got their names, and these elements are still few publications are present.
The structure of the periodic system represented by seven periods, with ten rows and eight groups. Each period starts with alkali metal and ends with noble gas. The exception is the first period starts with hydrogen, and a seventh incomplete period.
The periods are divided into small and large. Small periods (first, second, third) consists of a single horizontal row large (four, five, six) – two horizontal rows. The upper rows in the larger periods are called even, lower – odd.
In the sixth period of the table after lanthanum (number 57) there are 14 elements, similar properties for lanthanum, the lanthanides. They are made in the lower part of the table separately. The same applies to the actinides, after actinium (#89) and largely repeating its properties.
Elements in groups show the same higher valence in oxides and other compounds, and this value corresponds to the group number. Main groups contain elements of small and large periods, the side – just big. Top to bottom the metallic properties are intensified, non – weakened. All the atoms of the side sub-groups – metals.
With increasing atomic mass and observed periodic variation of properties of elements and their compounds. This metallicity and nemetallicheskie of atoms, atomic radius and volume, ionization potential, electron affinity, electronegativity, oxidation States, physical properties of compounds (boiling point, melting point, density), their basicity, or acidity afternote.
How many elements in the modern periodic table
The periodic table graphically expresses which he discovered the periodic law. In the modern periodic table contains 112 chemical elements (last – Materi, Darmstadt, Rentgene and Copernici). According to recent reports, opened and the next 8 elements (up to 120, inclusive), but not all of them got their names, and these elements are still few publications are present.
Each item occupies a cell in the periodic table and has a sequence number corresponding to the charge of the nucleus of its atom.
How to build a periodic system
The structure of the periodic system represented by seven periods, with ten rows and eight groups. Each period starts with alkali metal and ends with noble gas. The exception is the first period starts with hydrogen, and a seventh incomplete period.
The periods are divided into small and large. Small periods (first, second, third) consists of a single horizontal row large (four, five, six) – two horizontal rows. The upper rows in the larger periods are called even, lower – odd.
In the sixth period of the table after lanthanum (number 57) there are 14 elements, similar properties for lanthanum, the lanthanides. They are made in the lower part of the table separately. The same applies to the actinides, after actinium (#89) and largely repeating its properties.
Even-numbered rows of large periods (4, 6, 8, 10) is filled just metals.
Elements in groups show the same higher valence in oxides and other compounds, and this value corresponds to the group number. Main groups contain elements of small and large periods, the side – just big. Top to bottom the metallic properties are intensified, non – weakened. All the atoms of the side sub-groups – metals.