You will need
- - 300 ml of water;
- - 200 g of copper sulfate (copper sulfate (II));
- - glass Cup or beaker with a volume of 0.5 l;
- thread;
- ceramic stand (wall tile);
- - sand bath;
- - Petri dish or small glass;
- - oilcloth;
- - rubber gloves.
Instruction
1
First, make sure that the experiment will be conducted in a safe environment, especially for small children. Restrict their access to hot surfaces, glass and sharp objects, chemical reagents and solutions. Protect work surface with wax cloth, and his hands thin rubber gloves.

2
In a glass Cup or beaker pour 300 ml of water, add 200 g of copper sulfate. Put the beaker on the sand bath, turn on the heating. From time to time an aqueous solution of copper sulfate must be carefully stirred until completely dissolved its dry component. To heat the solution to approximately 80 degrees. While the solution is heated to prepare a seed: tie a string to a small piece of sulphate of copper, and the thread attach a long wooden skewer, a glass rod or a plastic rod from a ball pen.

3
Remove the beaker from the sand bath, putting it on a prepared ceramic tile (the stand). Observe precautions careless handling of a hot vessel you can to break it, to cut and to burn. Let the solution of copper sulphate to cool slightly, then place the prepared seed. Please note that it may dissolve, but the experiment is not affected. With the gradual cooling of the solution on the thread will form a small blue crystalof copper sulfate, however, the number of them will precipitate. This is due to the fact that upon contact with the cold tile surface of the heated glass beaker (the beaker) will be cooled much faster, and with it, respectively, and the solution.

4
Remove the filament from the crystalby ICA from a fully cooled solution again and repeat the procedure. Stand the beaker on a sand bath and heat the solution to dissolve the precipitate of copper sulphate. Carefully follow the process, in any case do not let the solution boil. Turn off the heater, without removing the glass from the sand. Cover the glass with glass or Petri dish and allow the solution to cool slightly.
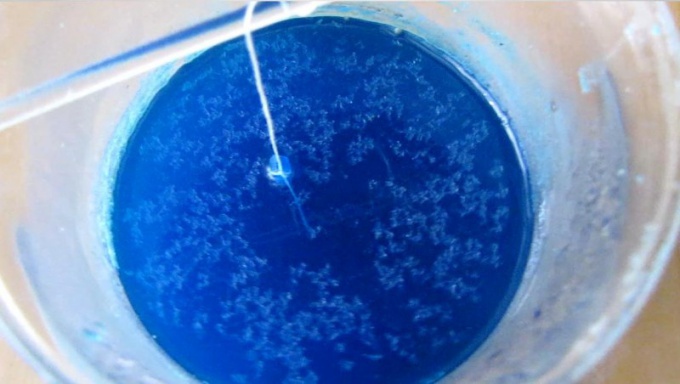
5
Carefully place the thread with the crystals in the solution, cover the beaker and leave overnight. In the morning you will find that the thread formed blue crystals with a size of 4-5 cm in the Experiment for growing a magical crystaland finished!

6
Do not pour the remaining solution of copper sulfate. It may be useful to you for growing the same crystal, but much larger. To do this, carefully pour the solution into a small glass jar, place a thread on a seed vessel and cover with a clean gauze pad or filter paper. The choice of priming in this case is very important: a crystalthat has external damage or defects, will give their "inherited" the future of the fruit of the experiment.

Useful advice
Ask the child to periodically look in the jar and check the status of a growing crystal, it is safe and very fascinating.




