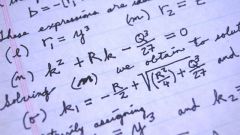You will need
- - the mass of the solute;
- - the mass of the solution.
Instruction
1
Mass fraction, it is the same percentage concentration is a dimensionless quantity, which is equal to the ratio of the mass solute to the total weight of the liquid. Most often it is expressed as a percentage, which you multiply the resulting ratio by one hundred. Formula percentage concentration can be recorded in the following way: ω = m in-VA/m R-RA*100%. The first value is the mass of the substance, and the second mass of the solution as a whole.
2
Often in the task given percentage concentration of a substance, based on which you want to find the mass of substance / mass of solution. It is very simple, just need to convert the original formula. To find the mass of a substance it will be as follows: m VA = m R-RA* ω/100. The mass of the solution can be found as follows: divide the mass of the substance in percent concentration and then multiply the result by one hundred. The unit mass of a substance and the mass of solution – grams.
3
To find the percentage concentration if the solution was obtained with the use of hydrated, you should use a different solution algorithm. The crystalline has the structure of IU(x)-TA(y)*nH2O. In the problem statement, which appears as a crystalline, should contain information about the mass of the hydrated and the dry mass metal-x-acid-y. In this case, the percentage concentration will be equal to the mass of the solution multiplied by the molar mass of hydrated divided by mass of crystalline, multiplied with the ratio dry substance and the molar mass of the anhydrous substance.
Useful advice
Counting the weight of the solution, note that it consists of two quantities: the mass of solvent and mass of a substance dissolved in it. The omission of this fact is a large number of errors during these tasks.