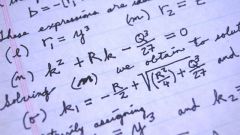You will need
- calculator.
Instruction
1
Any solution consists of a solute and solvent. In most cases the solvent is water. To calculate the percentage concentration (or mass fraction of the solute), you must use the formula:W = m (solute) / m (solution) x 100% W – mass fraction of solute (or percent concentration), %From the same formula, we can deduce the mass of solute if you know the mass of the solution and percent concentration of a solution.
2
Example No. 1. Calculate the mass fraction (percentage) of salt (NaCl) if the mass of (NaCl) 5 g, and the mass of the solution (NaCl) 100 g. In this problem, it remains only to substitute in the formula proposed in the condition parameters:W = m (b.-VA) / m (R-RA) x 100 % W (NaCl) = m (NaCl) / m (solution NaCl) x 100 % W (NaCl) = 5 g / 100 g x 100 % = 5 %Answer: W (NaCl) = 5 %
3
Example No. 2. Calculate the mass percent ( % ) potassium bromide (KBr), if the mass of salt (KBr) 10 g, and the weight of water, 190 g Before using formula to calculate percentage concentration, calculate the mass of the solution, which consists of water and dissolved substances:m (solution) = m (solute) + m (water) Consequently:m (R-RA KBr) = 10 grams + 190 grams = 200 godstate in the basic formula are found and specified in the condition parameters:W = m (b.-VA) / m (R-RA) x 100 % W (KBr) = m (KBr) / m (solution KBr) x 100 % W (KBr) = 10 g / 200 g x 100 % = 5% Answer: W (KBr) = 5 %
4
Example No. 3. Calculate the percentage concentration of acetic acid (CH3COOH), if the mass of the acid (CH3COOH) and 30 g, and the mass of water 170 g. Calculate the mass of the solution, which consists of water and acetic acid:m (solution) = m (solute) + m (water) Consequently:m (R-RA CH3COOH) = 30 g + 170 g = 200 godstate in the formula of required parameters:W = m (b.-VA) / m (R-RA) x 100 %W (CH3COOH) = m (CH3COOH) / m (solution CH3COOH) x 100 % W (CH3COOH) = 30 g / 200 g x 100 % = 15% Answer: W (CH3COOH) = 15 %