If the water container is left open, then after a certain period of time all the liquid from it evaporates. Evaporation – the physical process of transition of a substance from liquid to gaseous state. In the water, as in any other fluid molecules, the kinetic energy which allows them to overcome the intermolecular attraction. These molecules with the power are dispersed and fly to the surface. Therefore, if a glass of water, cover with a paper towel, then after a while it gets a little wet. But the evaporation of water under different conditions occurs with different intensity. Key physical characteristics that affect the speed of the flow of this process and its duration, are the density, temperature, surface area, presence of wind.The greater the density, the closer to each other are molecules. So, it is more difficult to overcome intermolecular attraction, and they are in much smaller quantities out to the surface. If you place two fluids of different densities (for example, water and methyl alcohol) in the same conditions, the faster it will evaporate the same, the density of which is less. Water density 0,99 g/cm3, and the density of methyl alcohol is 0,79 g/cm3. Therefore, the methanol will evaporate faster. An equally important factor affecting the water evaporation rate is temperature. As already mentioned, the evaporation occurs at any temperature, but it increase the speed of the molecules increases, and they are in greater numbers are beginning to leave the liquid. So burning water evaporates faster than cold.The rate of evaporation of water depends on the area of its surface. The water poured into a bottle with a narrow neck will evaporate slowly, because the emitted molecules will settle on the tapered top of the bottle walls and to roll back. And water molecules, located in the saucer, will freely leave the liquid.The evaporation process is significantly faster if above the surface at which evaporation occurs, move air flow. The fact that in addition to the release of molecules from the liquid they are back. And the stronger circulation of the air, the less molecules, sinking, fall back into the water. So, its volume will decrease rapidly.
Why does water evaporate?
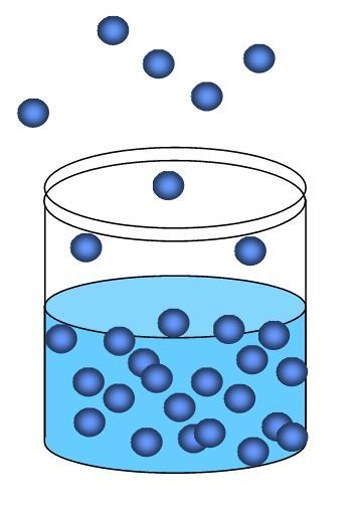
Evaporation is a natural physical process, due to the constant movement of molecules in liquids. It is important to note that water evaporation occurs at any temperature environment.
Is the advice useful?
