You will need
- - handle;
- paper for records;
- - the periodic system of elements (periodic table).
Instruction
1
The electrons in the atom occupy the available orbitals in the sequence, called the scale of energy:1s / 2s, 2p / 3s, 3p / 4s 3d 4p / 5s, 4d, 5p / 6s, 4d, 5d, 6p / 7s, 5f, 6d, 7p. On one orbital can accommodate two electrons with opposite spin rotation directions.
2
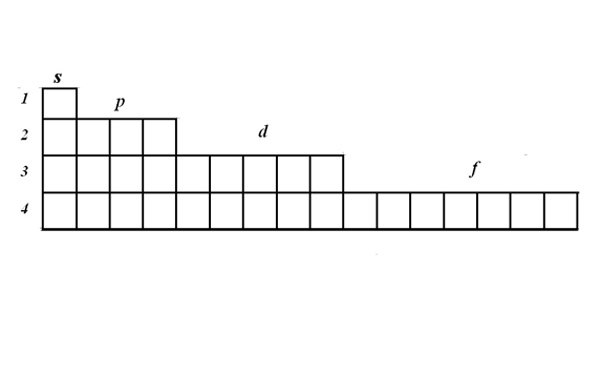
The structure of the electron shells Express through a graphical electronic formulas. To write formulas use the matrix. In one cell can accommodate one or two electrons with opposite spins. Electrons are represented by arrows. The matrix demonstrates that the s-orbital can accommodate two electrons, p orbitals 6, d – 10, f -14.
3
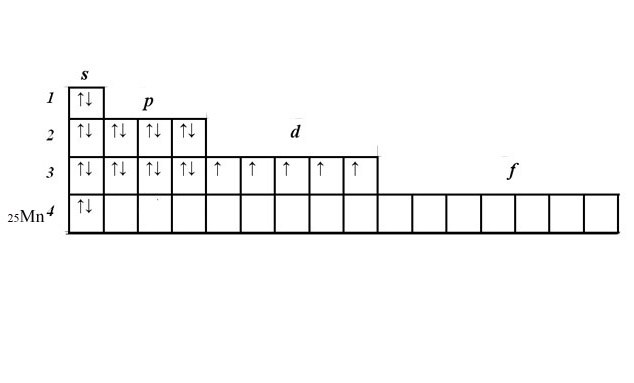
Consider the principle of making the electron-a graphic formula on the example of manganese. Find manganese in the periodic table. Its number 25 means 25 in the atom of electrons, is an element of the fourth period.
4
Record the serial number and the element symbol next to the matrix. In accordance with the scale of energy consistently fill in the 1s, 2s, 2p, 3s, 3p, 4s levels, typing for two electrons in a cell. Get 2+2+6+2+6+2=20 electrons. These levels are full.
5
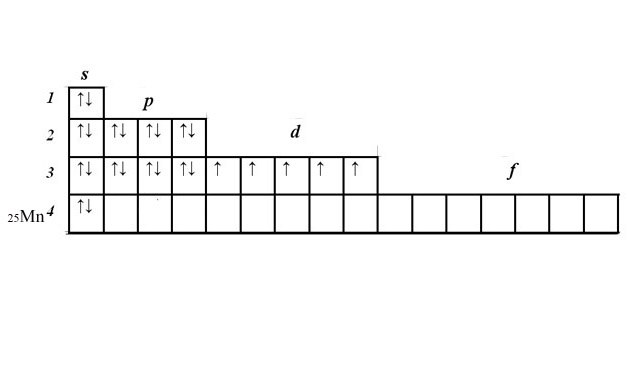
You still have five electrons and unfilled 3d level. Place the electrons in the cells of a d-sublevel, starting from the left. Electrons with the same spin place in the cells of the first one. If all the cells are filled starting from the left, add the second electron with opposite spin. The five manganese d-electrons, are located one in each cell.
6
Electron-graphic formula clearly show the number of unpaired electrons that determine the valency.
Note
Remember that chemistry is the science of exceptions. The atoms of auxiliary groups of the Periodic system there can be "leakage" of electrons. For example, chrome with serial number 24 is one of the electrons from the 4s-level moves into the cell d-level. A similar effect is, of molybdenum, niobium, etc. in addition, there is the concept of the excited state of the atom, when electrons are paired steamed and move on to the neighbouring orbitals. Therefore, in the preparation of electron-graphic formula of the elements of the fifth and subsequent periods secondary subgroups refer to the guide.