Instruction
1
Anode – electrode at which occurs the oxidation reaction. And the electrode at which recovery occurs is called the cathode.
2
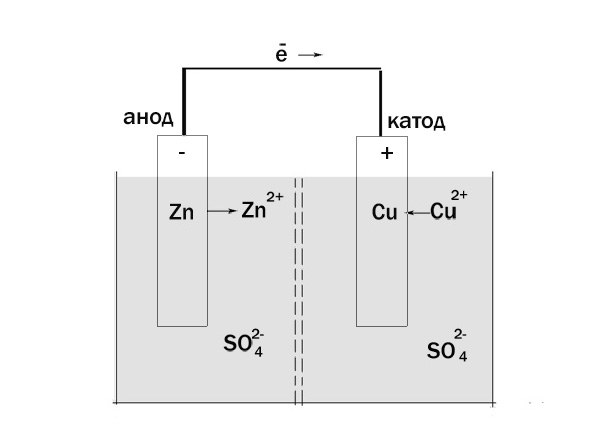
Take for example a galvanic element Jacobi-Daniel. It consists of zinc electrode dipped in a solution of zinc sulfate and a copper electrode in copper sulfate solution. Solutions in contact with each other, but not mixed – it is provided between the porous partition.
3
The zinc electrode are oxidized, it gives up its electrons, which move the external circuit to the copper electrode. Copper ions from the solution accept electrons СиЅО4 and restored at a copper electrode. Thus, in a galvanic cell the anode is negatively charged and the cathode is positive.
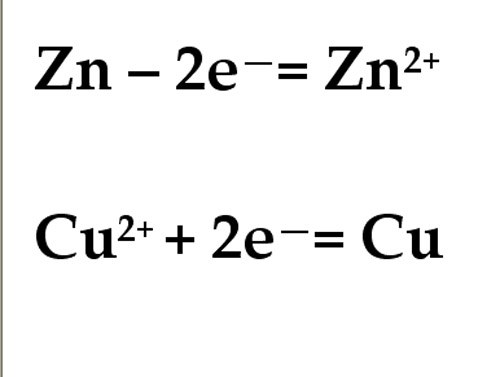
4
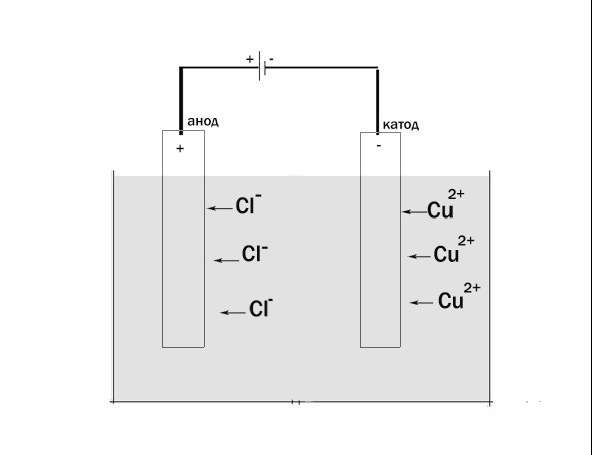
Now consider the process of electrolysis. Installation for electrolysis comprises a vessel with a solution or melt of the electrolyte, which lowered the two electrodes connected to a DC power source. A negatively charged electrode is the cathode, it is recovery. The anode in this case, the electrode connected to the positive pole. It is oxidation.
5
For example, in the electrolysis solution СuCl2 occurs at the anode of the recovery of copper. The cathode is the oxidation of chlorine.
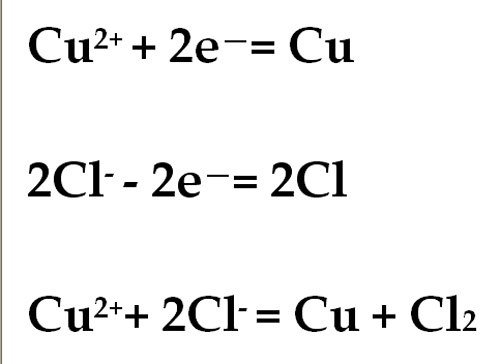
6
Therefore, please note that the anode is not always a negative electrode, as the cathode is not in all cases has a positive charge. Factor determining electrode, is occurring in this oxidation or reduction process.


