What is acid-basic properties
The main properties are metals, their oxides and hydroxides. Acid properties are the non-metals, their salts, acids and anhydrides. There are also amphoteric elements, can show both acidic and basic properties. Zinc, aluminum and chromium are among the representatives of the amphoteric elements. Alkaline and alkaline-earth metals exhibit the typical basic properties as sulfur, chlorine and nitrogen acid.
Thus, the reaction of the oxides with water, depending on the properties of the main element, obtained either base or hydroxide, or acid.
For example:
SO3+H2O=H2SO4 is a manifestation of acidic properties;
CaO+H2O=Ca(OH)2 - a manifestation of the basic properties;
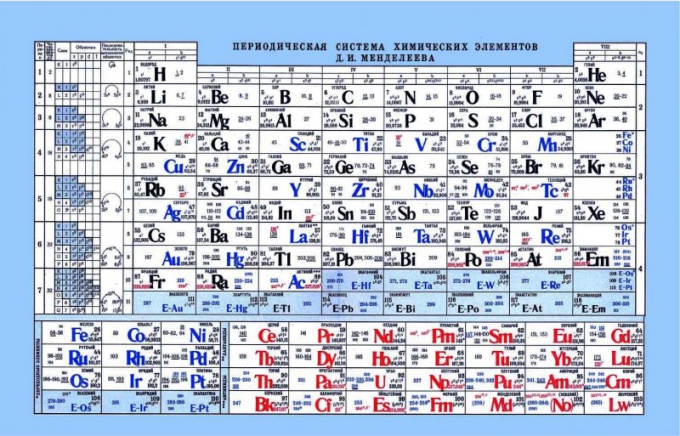
Mendeleev's periodic table, as an indicator of acid-base properties
The periodic table can help in determining the acid-base properties of elements. If you look at the periodic table, we can see a pattern, horizontally from left to right are enhanced or non-acid properties. Respectively closer to the left are metals, center, amphoteric elements, and nonmetals on the right. If you look at the electrons and their attraction to the nucleus, it is noticeable that in the left part elements have the weak charge of the nucleus, and electrons are in s-level. As a result, such elements easier to give an electron than elements on the right side. Nonmetals have relatively high charge of the nucleus. Thus complicated the return of free electrons. Such elements are easier to attach to itself the electrons, showing acidic properties.
Three theories to determine the properties
There are three approaches that define what properties has connection: proton theory of Bronsted-Lowry, aprotic electronic theory of Lewis, the theory of Arrhenius.
According to the proton theory of acid properties of compounds able to give up their protons. Such compounds have been named by donors. But the main properties are the ability to accept or to attach a proton.
Aprotic approach implies that the acceptance and donation of protons to determine the acid-base properties is optional. The acid properties of this theory appear to have taken e a couple, as principal, on the contrary, to give this couple.
The theory of Arrhenius is the most relevant for the determination of acid-base properties. During the research it was proved that the acid properties are manifested when under the dissociation of aqueous solutions of a chemical compound is separated into anions and hydrogen ions and basic properties of the cations and hydroxide ions.