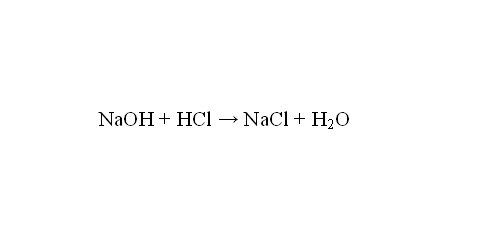You will need
- - handle;
- paper for records;
- calculator;
- - The periodic system of chemical elements (the periodic table).
Instruction
1
In the aforementioned method, measured volumes of the two which entered into reaction solutions, one of which is analyzed, and the second titrant or titrated solution of known concentration. For titrant, there is the concept of conditional title or caption at the designated substance. Is the amount of analyte, titrated with 1 ml of solution. In the course of chemistry there are several types of problems on the calculation of titer of the solution.
2
In the first type of tasks you will need to translate the concentration of the solution from other units in titer. Concentration is the ratio of the magnitude of the dissolved substance, given mass, number of moles, the volume, the amount of solution or solvent. When solving rely on that to determine the titer of the original data it is necessary to mass of solute and volume of solution in which it is located.
3
Example 1: determine the titer of 15% sulfuric acid solution. Solution density of 1.10 g/ml. the concentration of the solution expressed in mass fraction of the substance. Mass fraction is the ratio of the masses of the solute and solution. Calculate the mass of one liter of a solution of 1100 grams. Determine the mass content of sulfuric acid: 1100*0,15=165g. Calculate the titer of the solution: 165 g/1000 ml=0,165 g/ml.
4
Example 2: you want to find the titer of 0.15 n sulfuric acid solution. Normality of a solution is the number of equivalent of solute per liter of solution, the unit mol – EQ/l Equivalent is the amount of substance equivalent to 1 pray of hydrogen ions in chemical reactions. One litre of a solution contains 0.15 mole equivalent of sulfuric acid.
5
Using the periodic table, find the molar mass of H2SO4 is 98 g/mol. Equivalent of sulfuric acid is 1/2. Calculate the molar equivalent weight of H2SO4: 98/2=49 g/mol. Find what you weigh 0.15 mole equivalent of sulfuric acid: 0,15*49=of 7.35 g. Determine the titer of a solution of 7.36 g/1000ml=0,00736 g/ml.
6
In the second type of task it is necessary to find the conditional titer. For the solution calculate the initial values of the mass of solute and volume of solution with which it is reacted.
7
Example 3: calculate the titre solution of 0.1 n AgNO3 solution by NaCl. Equivalents of AgNO3 and NaCl are equal to one. Find the molar mass of NaCl of 58.5 g/mol. Find the number of silver nitrate in 1 ml of a solution of 0.0001 mol. Therefore, the amount of sodium chloride that reacts with 1 ml of a solution of 0.0001 mol. Multiply the molar mass of NaCl on the quantity of the substance and will receive a conditional titer of the silver nitrate solution — 0,000585 g/ml is the mass of NaCl reacting with 1 ml of AgNO3 solution.
8
The third type of tasks is the calculation of titer of the solution of the values obtained by titrimetric method. For their decision on the reaction equation analysis. From it find out in what proportion the substances react with each other.
9
Example 4: determine the titer of HCl solution if 20 ml of neutralization of the acid it took 18 ml of 0.13 n NaOH solution. Equivalents of HCl and NaOH is equal to one. Find the number of sodium chloride in 18 ml: 0,13*0,018=0,00234 mol. Therefore, the amount of unreacted hydrochloric acid is also will 0,00234 mol. Calculate the molar mass of HCl is 36.5 g/mol. Find the mass of the obtained the amount of hydrochloric acid: 0,00234*36,5=0,08541, This mass of matter contained in a 20 ml solution. Find the titer of the solution: 0,08541/20=0,0042705 g/ml.