General concepts
From the point of view of science, the absolute pressure is the pressure in the system to pressure in a vacuum. The most common expression for the absolute pressure is the sum of the sensor system and atmospheric pressure. The expression takes the form:
Absolute pressure = Gauge pressure + atmospheric pressure.
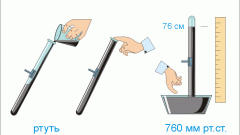
Atmospheric pressure is defined as the ambient air pressure on the Earth's surface. This value is not fixed or constant and may vary depending on temperature, altitude and humidity.
Gauge pressure represents the pressure in the system, which was measured using a measuring device. These devices or sensors can be classified according to structural features. The most common types are sensors based on elastic elements, sensors with the fluid column and electrical appliances. If the sensor does not take into account the pressure of the atmosphere in his testimony, the calculation of absolute pressure made by hand.
Units of measurement and practical application
In practice, absolute and gauge pressure are not the same characteristics of the system. Therefore, each of them uses its own designation. The most common technique is to add indexes. After the letter denoting the absolute pressure, put the index "a", and after gauge – "m".
Such designations are most often used in engineering calculations. In their implementation it is necessary to use a proper marking pressure to avoid mistakes. The difference between absolute and gauge pressure is much more pronounced in the case when the atmospheric pressure has the same order of magnitude as the gauge.
Neglect atmospheric component of the absolute pressure in the calculations also leads to serious errors in the design. This can be demonstrated by examining a closed cylinder with an ideal gas at a temperature of 25oC and a volume of 1 cubic meter. If the pressure gauge on the cylinder shows a pressure of 100 KPa, and the pressure of the atmosphere is not taken into account, the estimated number of gas moles in the cylinder is approximately 40,34.
When the atmospheric pressure is 100 KPa, the absolute pressure is actually equal to 200 KPa and the correct number of moles of gas will 80,68. The actual number of moles of gas will be two times more compared to the original calculation. This example shows the importance of using the correct algorithm for calculating the pressure.