You will need
- Handbook of physical quantities, the calculator.
Instruction
1
Thus, all long been no secret that the density of a substance, whether liquid or solid aggregate state can be calculated as mass divided by volume. That is, in order to experimentally determine the density of ordinary liquid water, you need to:1) Take a measuring cylinder, weigh it.
2) Pour water into it, to fix its occupied volume.
3) Weigh the cylinder with water.
4) Calculate the difference of the masses, giving you a lot of water.
5) Calculate the density of the known formula
2) Pour water into it, to fix its occupied volume.
3) Weigh the cylinder with water.
4) Calculate the difference of the masses, giving you a lot of water.
5) Calculate the density of the known formula
2
However, scientists noticed that the density values differ at different temperatures. But the most surprising thing, according to what law this change takes place. So far on this phenomenon puzzled scientists around the world. No one can unravel the mystery and answer the question: "Why is the density when heated from 0 to 3.98 increases, and then decreases to 3.98?" A couple of years ago the Japanese physicist, Masakazu Matsumoto suggested a model of the molecular structure of water. According to this theory, creates some polygonal microabrasive - vitrite, which, in turn, dominated by the phenomenon of the lengthening of the hydrogen bonds and shrink the molecules of water. However, this theory is still not confirmed experimentally. A graph of the dependence of density on temperature are presented below. To use it you need to:1) Find the desired temperature value on the corresponding axis.
2) Drop a perpendicular on a graph. Mark the intersection of a line and function.
3) From the resulting points to draw a line parallel to the axis of the temperature on the axis density. The point of intersection is the desired value.Example: Let the temperature of the water 4 degrees, then the densityafter building, is equal to 1 g/cm^3. Both of these values are approximate.
2) Drop a perpendicular on a graph. Mark the intersection of a line and function.
3) From the resulting points to draw a line parallel to the axis of the temperature on the axis density. The point of intersection is the desired value.Example: Let the temperature of the water 4 degrees, then the densityafter building, is equal to 1 g/cm^3. Both of these values are approximate.
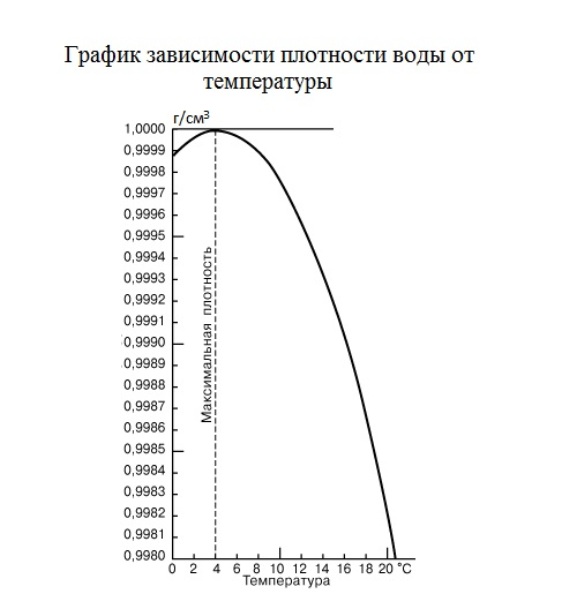
3
To determine a more accurate density values, it is necessary to use a table. If there is no data when the desired temperature value, then: 1) Find the values between which is the desired result. For better understanding, consider the example immediately. Let the desired density of water at a temperature of 65 degrees. It is between 60 and 70.
2) Draw a coordinate plane. Set the axis of abscissa as the temperature, the y-axis as density. Note on the chart of known points (A and B). Connect them direct.
3) Drop a perpendicular from the desired values of temperature on the period obtained above, mark it as point C.
4) Mark the points D, E, F, as shown on the chart.
5) Now clearly shows that the triangles ADB and AFC are similar. Then the relation is valid:
AD/AF=DB/EF, therefore:
(0,98318-0,97771)/(0,98318-x)=(70-60)/(65-60);
0,00547/(0,98318-x)=2
1,96636-2=0,00547
x=0,980445
Accordingly, the density of water at 65 degrees equals 0,980445 g/cm^3
This method of finding the values is called interpolation.
2) Draw a coordinate plane. Set the axis of abscissa as the temperature, the y-axis as density. Note on the chart of known points (A and B). Connect them direct.
3) Drop a perpendicular from the desired values of temperature on the period obtained above, mark it as point C.
4) Mark the points D, E, F, as shown on the chart.
5) Now clearly shows that the triangles ADB and AFC are similar. Then the relation is valid:
AD/AF=DB/EF, therefore:
(0,98318-0,97771)/(0,98318-x)=(70-60)/(65-60);
0,00547/(0,98318-x)=2
1,96636-2=0,00547
x=0,980445
Accordingly, the density of water at 65 degrees equals 0,980445 g/cm^3
This method of finding the values is called interpolation.
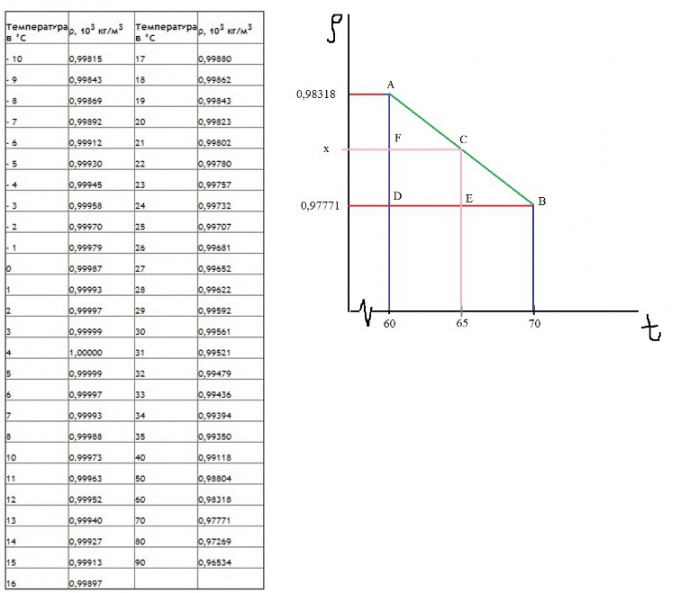
Note
Pay attention to the dimensions.
Useful advice
Carefully carry out all the calculations. Remember that the data in this table are given for ordinary water. From salt water the density is greater.


