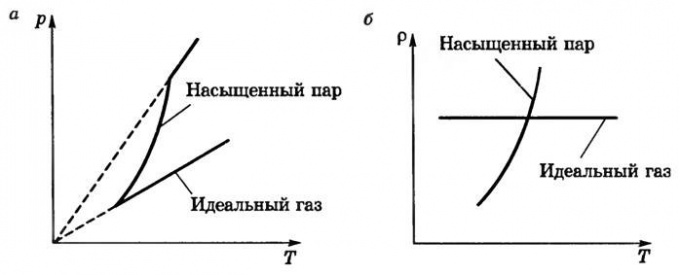
Since saturated steam is a single component thermodynamic equilibrium the system is homogeneous in composition, but of different phase fractions of a substance, the understanding of the influence of certain physical factors on the magnitude of the generated pressure allow them to use this knowledge in practical activities, for example, when determining the rate of burnout of certain liquids in case of fire, etc.
The vapor pressure becomes greater, the more temperature increases. The change in values is not directly proportional to, and is much faster. This is due to the fact that an increase in temperature accelerates the movement of molecules relative to each other and it is easier to overcome the forces of mutual attraction and go in a different phase, i.e., the number of molecules in the liquid state decreases and gas increases until, until all the liquid turns into vapor. This increasing pressure causes lifting of the lid in the pot or the kettle when it begins to boil the water.
The magnitude of the steam pressure is also influenced by the number passed in gaseous state molecules, as their number determines the weight of the resulting vapor in a closed vessel. This value is not constant, since the temperature difference between the bottom of the vessel and closing its lid constantly occur two opposite processes – evaporation and condensation.
As for every substance at a certain temperature there are known indicators of the transition of a number of molecules from one phase state of matter to another, we change the value of the steam pressure by changing the volume of the vessel. So, the same volume of water, for example 0.5 liters, will create a different value of pressure in a five-liter canister liter kettle.
The determining factor for determining the reference pressure saturated steam at constant scope and gradual increase in temperature is the molecular structure of the liquid is being heated. Thus, the value for acetone, alcohol, and normal water will be significantly different from each other.
To see the process of boiling the liquid it is necessary not only to bring the vapor pressure up to certain limits, but also to correlate this value with the external atmospheric pressure as the boiling process is possible only in the case where the pressure outside is greater than the pressure inside the vessel.
Dependence of pressure of saturated steam temperature
The vapor pressure becomes greater, the more temperature increases. The change in values is not directly proportional to, and is much faster. This is due to the fact that an increase in temperature accelerates the movement of molecules relative to each other and it is easier to overcome the forces of mutual attraction and go in a different phase, i.e., the number of molecules in the liquid state decreases and gas increases until, until all the liquid turns into vapor. This increasing pressure causes lifting of the lid in the pot or the kettle when it begins to boil the water.
The dependence of the steam pressure on the other factors
The magnitude of the steam pressure is also influenced by the number passed in gaseous state molecules, as their number determines the weight of the resulting vapor in a closed vessel. This value is not constant, since the temperature difference between the bottom of the vessel and closing its lid constantly occur two opposite processes – evaporation and condensation.
As for every substance at a certain temperature there are known indicators of the transition of a number of molecules from one phase state of matter to another, we change the value of the steam pressure by changing the volume of the vessel. So, the same volume of water, for example 0.5 liters, will create a different value of pressure in a five-liter canister liter kettle.
The determining factor for determining the reference pressure saturated steam at constant scope and gradual increase in temperature is the molecular structure of the liquid is being heated. Thus, the value for acetone, alcohol, and normal water will be significantly different from each other.
To see the process of boiling the liquid it is necessary not only to bring the vapor pressure up to certain limits, but also to correlate this value with the external atmospheric pressure as the boiling process is possible only in the case where the pressure outside is greater than the pressure inside the vessel.