You will need
- - handle;
- paper for records;
- calculator.
Instruction
1
A constant equilibrium can be expressed using equilibrium concentrations of the reaction participants, i.e. the concentration of substances at a time when the speed of the forward reaction equals the rate of reverse. Given the reversible reaction of substances A and b In certain conditions with the formation of substances: na+mB ↔ zС, where n, m, z – the coefficients in the equation reactions. A constant equilibrium can be expressed by: Kc = [C]^z/ ([A]^n*[B]^m), where [C], [A], [B] is the equilibrium concentration of substances..
2
In the first type of task you want to define the constant of equilibrium of the equilibrium concentrations. Equilibrium concentrations may not be asked directly. When solution first write down the reaction equation, place coefficients.
3
Example: nitrogen monoxide under certain conditions, reacts with oxygen c NO2 formation. The initial concentrations of NO and O2 – 18 mol/l and 10 mol/L. it is Known that methane 60% O2. You want to find the constant of equilibrium reaction.
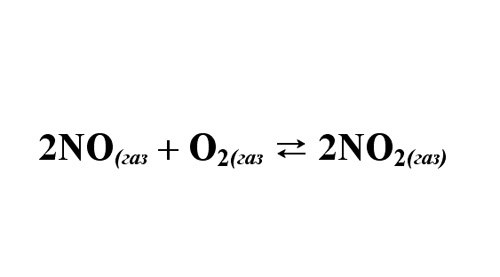
4
Write down the reaction equation, place coefficients. Please note, the proportions in which substances react. Calculate the concentration of O2, which entered into reaction: 10моль*0,6 = 6 mol/L. From the reaction equations, find the concentration of unreacted NO – 12 mol/L. the NO2 concentration is 12 mol/L.
5
Determine the amount of unreacted NO: 18-12 = 6 mol. And unreacted oxygen: 10-6 = 4 mol. Calculate the constant of equilibrium: KS = 12^2/(6^2*4) = 1.
6
If the clause specified the rate constants of direct and reverse reactions, find the constant of equilibrium from the relation: K = k1/k2 where k1, k2 – the rate constants of the forward and reverse chemical reactions.
7
In an isothermal process and Isobaric process a constant equilibrium can be found from the equation of the standard changes of Gibbs energy: ΔGр- = - RT*lnKc = -8,31 T*2,3 lgKc, where R is the universal gas constant, equal to 8,31; T – reaction temperature, K; lnKc – the natural logarithm of the constants of equilibrium. For convenience, convert it into decimal lgKc by multiplying by a factor of 2.3.
8
To determine the change in standard Gibbs energy of reaction, you can from the equation for isothermal Isobaric process: ΔG = ΔH – T ΔS, where T is the reaction temperature, K; ∆ H - enthalpy, kJ/mol; ΔS is entropy, j/(mol-deg). The value of enthalpy and entropy for 1 mole of basic chemical compounds at a temperature of 25 ° C are given in reference literature. If the reaction temperature differs from 25 ° C, the values of enthalpy and entropy should be given in the problem statement.
9
Magnitude of the ΔG of the reaction at 25 ° C you can find the folding potentials of education ΔGобр each of the reaction products and subtracting from the sum ΔGобр starting materials. The potential values of education 25oC for 1 mole of various substances are given in reference tables.
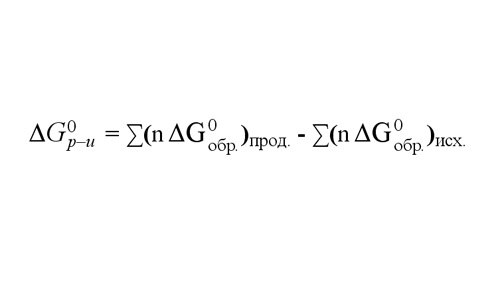
Note
In the case that the participants in the reaction are in different States of aggregation, in the formula for determination of the equilibrium constant includes the concentrations of substances in more a mobile (gas or liquid) state.