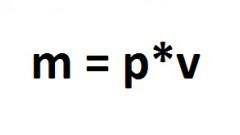You will need
- - a substance;
- - Internet;
- - the periodic table.
Instruction
1
Determine what type of mass you need to calculate: average, molecular or molar. Find the formula of a chemical compound, a lot of which you need to calculate. If it is not in the task, start a search by name on the Internet.
2
Count the number of chemical elements in the molecule you are interested in substance. For example, aluminum sulfate Al2(SO4)3 consists of two atoms of aluminium, three atoms of sulphur and twelve oxygen atoms.
3
Open the periodic table. The atomic mass listed for each element under its letter, the exact number of tables in the calculations round to the nearest integer. So, atomic mass of aluminium = 27 (26,98154 on the table), sulphur = 32 (of 32.06 in the table), oxygen = 16 (15,9994). Write down the atomic mass of each element. Molecular mass equals the sum of the atomic masses of all the elements of the substance, taking into account their number in the connection.
4
Fold the atomic mass by multiplying each of them by the number of the chemical element in the formula, you will get the molecular mass of the substance:
2Al +3S+12О = 2*27+3*32+12*16 = 342
Molecular mass has no units.
2Al +3S+12О = 2*27+3*32+12*16 = 342
Molecular mass has no units.
5
To determine the conventional mass of a certain number of substances need to know the molar mass (mass of one mole of this compound, it is expressed in grams per mole, g/mol, and is directly related to molecular weight). To do this, to the obtained value of molecular weight just append "g/mol". That is, molar mass of aluminium sulphate is 342 g/mol.
6
Molar and normal weight are interrelated by the formula: m = ? * M , where M is the usual mass expressed in grams ? - the amount of substance in moles, M is molar mass in g/mol. Multiply the molar mass by the number of moles and get a lot of substance. So, 1 mole of aluminum sulfate weighs 342 grams, 2 mole - 684 grams, etc.
7
If you know the amount of substance in moles and weight, molar mass calculate the formula M = M / ?.